Volume 39, Issue 1 ⦁ Pages: 87-96
Abstract
Binge drinking is a pattern of alcohol drinking that raises a person’s blood alcohol concentration to at least .08%, which amounts to consuming five alcoholic drinks for men and four alcoholic drinks for women in about 2 hours. It is the most common form of alcohol misuse in adolescents and young adults. Heavy drinking includes the same criterion as binge drinking, but with higher frequency (i.e., 5 or more days in the past 30 days). Although binge drinking or heavy drinking alone is insufficient to meet the criteria for an alcohol use disorder (AUD) diagnosis, there are neurobiological changes, as well as an increased risk of developing an AUD later in life, associated with this form of alcohol misuse. This review describes the recent neuroimaging findings in binge drinking and heavy-drinking adolescents and young adults, a developmental period during which significant neuromaturation occurs.
It has been well established that the brain undergoes significant maturation during adolescence that continues into young adulthood.1 Studies using structural magnetic resonance imaging have described linear and nonlinear changes in cortical gray-matter volume and thickness2-5 and increases in white-matter volume and integrity2,6-9 occurring during development. Gray-matter volume peaks earlier in females (i.e., around age 11) than in males (i.e., around age 12) and declines during adolescence due to pruning of unused synaptic connections in order to promote efficient communication between neurons.6 Furthermore, gray matter has been shown to reach earlier maturation in the sensorimotor cortices, whereas the frontal and temporal cortices mature later in development.4 The prefrontal cortex, which is central to executive control, matures later compared with earlier developing limbic structures thought to be more involved in reward and emotional processing.6,10,11 The asynchronous development of the prefrontal cortex and emotional and reward circuitry has been hypothesized to result in increased risk-taking behavior during adolescence, such as alcohol use.12-15 This is especially of concern because ongoing neurodevelopment may render the adolescent brain particularly vulnerable to the neurotoxic effects of alcohol, as has been shown repeatedly in animal models. 16-19
Binge drinking is a pattern of alcohol drinking that raises a person’s blood alcohol concentration to at least .08%, which amounts to consuming five alcoholic drinks for men and four alcoholic drinks for women in about 2 hours.20 It is the most common pattern of alcohol consumption in adolescents and young adults. As of 2014, 1.5 million adolescents ages 12 to 17 (6.1%) and 13.2 million young adults ages 18 to 25 (37.7%) in the United States reported binge drinking.21 Heavy drinking includes the same criterion as binge drinking, but with higher frequency (i.e., 5 or more days in the past 30 days).21 In the National Survey on Drug Use and Health, 257,000 adolescents (1%) and 3.8 million young adults (10.8%) reported heavy drinking.21 Although binge or heavy drinking alone is insufficient to meet criteria for an alcohol use disorder (AUD) diagnosis, there are neurobiological changes, as well as an increased risk of developing an AUD later in life, associated with this form of alcohol misuse.22 This article reviews neuroimaging studies assessing the effects of binge and heavy drinking on brain structure and function in adolescents. Studies in which participants met criteria for AUD were not included. Further, the age range included studies in adolescents and young adults, which extends up to a mean age of 25, because brain maturation continues to occur well into the late 20s.2
Effects on Brain Structure—Gray Matter
Volume
Cross-sectional studies in binge drinking adolescents and college-age individuals have demonstrated regions of both more and less gray-matter volume compared with nondrinking peers, with volumes often related to frequency and quantity of alcohol consumption. For example, a recent study found that adolescents and young adults who consumed moderate to high levels of alcohol had smaller total-brain, frontal-lobe, and temporal-lobe volumes than their nondrinking peers; however, they also found that a greater number of lifetime drinks was positively associated with greater temporal-lobe volume.9 In support of the notion that binge drinking is associated with lower gray-matter volume, a study of college-age binge drinkers found that higher Alcohol Use Disorders Identification Test (AUDIT) scores, indicative of greater reported frequency and quantity of alcohol consumption and alcohol-related problems, were associated with smaller frontal-lobe volumes.23 An association between alcohol use and smaller gray-matter volume also was supported by another study that identified smaller precuneus volumes in a group of college-age binge drinkers compared with alcohol-naïve controls.24 Further, greater AUDIT scores again were associated with smaller gray- matter volumes in the amygdala and hippocampus.24 Additionally, among binge drinking adolescents, greater peak number of drinks in the past 3 months was associated with decreased cerebellar gray-matter volume.25 Together, these findings suggest that binge drinking during development is associated with various regions of lower cortical, subcortical, and cerebellar brain volume, and that these changes often are associated with alcohol drinking characteristics.
Contrary to findings of smaller brain volumes, Howell and colleagues reported greater ventral striatal, thalamic, and lingual-gyrus volumes in college-age binge drinkers compared with control subjects.24 A study on binge drinking, college-age participants also found increased frontal, occipital, anterior cingulate cortex (ACC), and posterior cingulate cortex volumes compared with nondrinking control subjects.26 In this study, larger dorsolateral prefrontal cortex (DLPFC) volumes were positively associated with speed and quantity of alcohol consumption and negatively associated with age of onset of alcohol use.26 It is worth noting that these individuals reported binge drinking for a minimum of 3 years prior to neuroimaging sessions, suggesting that volumetric increases in regional gray matter may be associated with long-term binge drinking.
In addition to these disparate findings in gray-matter volume, sex-specific effects also have been observed in college-age binge drinkers. Kvamme and colleagues noted a significant sex-by-drinking status interaction in numerous prefrontal, parietal, temporal, and striatal regions, such that binge drinking males had smaller volumes than alcohol-naïve males, whereas binge drinking females had larger volumes than alcohol-naïve females.23 Although these sex-specific effects partially may explain the bidirectional effects seen in previous studies, there are likely many other factors that could contribute to these disparate findings, including the inability of cross-sectional designs to capture alterations in nonlinear developmental trajectories.2-5
To better address volume-related changes associated with drinking, longitudinal studies have begun to investigate gray-matter volume both before and after binge drinking. The first of such studies examined heavy-drinking adolescents with a baseline magnetic resonance imaging scan when the subjects were alcohol naïve and a follow-up scan approximately 3 years later, after binge drinking. At baseline, adolescents who later transitioned into heavy drinking had smaller ACC, posterior cingulate cortex, and inferior frontal gyrus (IFG) gray-matter volumes.27 Furthermore, heavy-drinking adolescents showed accelerated reductions in the thalamus/hypothalamus, inferior temporal gyrus, middle temporal gyrus (miTG), caudate, and brain stem, with greater lifetime alcohol use associated with a greater reduction in gray-matter volume in the left caudate and brainstem.27
A follow-up to this study that investigated gray-matter volumes in heavy-drinking adolescents at baseline and during multiple follow-ups found that heavy drinkers exhibited greater reductions in overall neocortex volume, as well as in frontal, lateral frontal, and temporal cortex volumes.28 Finally, Whelan and colleagues used machine-learning techniques to classify adolescents before and after initiation of binge drinking.29 They reported that before alcohol use, binge drinking adolescents had lower gray-matter volume in the superior frontal gyri (SFG) and greater volume in the premotor cortex compared to nondrinking control subjects. After alcohol initiation, however, smaller ventral medial prefrontal cortex (PFC) and IFG volumes were observed compared with nondrinking controls.29 Taken together, these findings suggest that binge drinking during development may result in accelerated decreases in gray-matter volume, above and beyond what is seen in typical maturation, likely caused by the neurotoxic effect of alcohol. It also is possible, based on evidence from cross-sectional studies in college-age individuals (described above), that a longer duration of alcohol use into young adulthood may result in greater gray-matter volumes in young adults who binge drink, potentially because of impaired synaptic pruning. Additional longitudinal studies with multiple time points will be necessary to elucidate alcohol’s effects on the full developmental trajectory across adolescence and young adulthood.
Cortical Thickness
Generally, studies investigating cortical thickness in binge drinking adolescents have supported findings of decreases in gray matter. Similar to their gray-matter volume findings noted above, Pfefferbaum and colleagues noted that alcohol-consuming adolescents had thinner total, frontal, temporal, and cingulate cortices than nondrinkers; moreover, the number of binge drinking episodes in the past year was negatively associated with frontal and parietal cortex thickness.9 This finding is in agreement with another cross-sectional study of young adults, which determined that binge drinkers had thinner cortical measures in the ACC and posterior cingulate cortex compared with light drinkers (i.e., consuming one or two drinks per week, but no binge episodes).30 Further, ACC cortical thickness was negatively correlated with the number of drinking occasions and number of drinks per occasion in the past 3 months, indicating that greater frequency and quantity of use is associated with thinner cortices.30
Similar to the volumetric study previously cited, sex-specific effects also have become apparent when investigating cortical thickness in binge drinking adolescents.23 A cross-sectional study in binge drinkers identified sex-by-drinking status interactions for cortical-thickness measures in four frontal regions (i.e., frontal pole, pars orbitalis, medial orbital frontal, and rostral anterior cingulate). Thus, binge drinking males had thinner cortices than alcohol-naïve control subjects, whereas binge drinking females had thicker cortices than alcohol-naïve control subjects.31 The directionality of these findings is consistent with those of Kvamme and colleagues.23 The findings suggest that during this particular window of development, alcohol may have differential effects for boys and girls, likely resulting from underlying sex differences in the rate and timing of synaptic pruning in adolescents.6
In a longitudinal investigation of the effects of binge drinking on cortical thickness, Luciana and colleagues found that adolescents who initiated alcohol use showed a significantly greater decrease in middle frontal gyrus (miFG) cortical thickness between baseline and revisit compared with adolescents who remained alcohol naïve,32 suggesting that alcohol has a neurotoxic effect on frontal lobe development. However, this study found no differences in cortical thickness prior to initiation of alcohol use, contrary to a subsequent study observing differences in baseline gray-matter volume.27 Other studies have investigated the effects of binge drinking on cortical thickness in a longitudinal manner, but without an alcohol-naïve baseline. Jacobus and colleagues examined cortical thickness over 3 years and found that concomitantly binge drinking and marijuana using adolescents had thicker cortices across time in five frontal, eight parietal, one temporal, and one occipital region compared with alcohol- and marijuana-naïve control subjects.33 Moreover, in three frontal regions, control subjects showed a decrease in cortical thickness across time, whereas concomitantly binge drinking and marijuana using adolescents did not. A prior study had suggested that these effects persisted following abstinence, because concomitantly binge drinking and marijuana using adolescents showed greater thickness in the ACC, medial temporal gyrus, lingual gyrus, and occipital cortex both before and after 28 days of monitored abstinence.34
Taken together, these studies suggest that, when combined with marijuana use, binge drinking may result in increases, as opposed to decreases, in cortical thickness, that these increases are cumulative with prolonged use, and that they persist even following a month of abstinence. Furthermore, although these studies contradict some literature,9,30,32 they may help provide an alternative explanation for the equivocal findings in gray-matter volume described above. In fact, in the longitudinal study by Squeglia and colleagues, although a greater number of lifetime alcohol-use occasions was associated with greater reductions in caudate and brainstem volume, a greater number of lifetime marijuana uses was associated with increases in caudate volume.27 This provides further evidence that although gray-matter volume and thickness typically decrease in binge drinking adolescents and young adults, concomitant marijuana use may result in observed increased volume and thickness.
Effects on Brain Structure—White Matter
Volume
As opposed to the varied findings in gray-matter volume, results in white-matter volume have been more parsimonious. Cross-sectional studies have shown that a greater number of lifetime drinks was associated with smaller central white-matter volume,9 and peak number of drinks during a binge episode in the past 3 months was associated with smaller cerebellar volumes.25 Longitudinal studies tell a similar story, with binge drinking adolescents showing reduced white-matter volumes both before27 and following initiation of binge drinking.28,32 Squeglia and colleagues found that heavy-drinking adolescents had lower baseline cerebellar white-matter volumes compared with control subjects, but the investigators identified no regions where white-matter volume changed differentially across time.27 However, in a follow-up study, heavy-drinking adolescents exhibited significantly attenuated white-matter growth in the pons and corpus callosum between baseline and follow-up scans, compared with controls.28 Luciana and colleagues reported similar findings, such that alcohol-naïve controls showed an increase in volume in white-matter regions of the precentral gyrus, miTG, SFG, and lingual gyrus between baseline and follow-up, whereas binge drinking adolescents did not.32 Taken together, these observations suggest that reduced white-matter volume may precede alcohol use, and that alcohol use during adolescence attenuates the typical maturational increase in white-matter volume observed in adolescence in a dose-related fashion.2,6-8
Microstructure
Varied differences in white-matter microstructure have been observed between binge drinking adolescents (with and without concomitant marijuana use) and non–alcohol using controls. First, a cross-sectional diffusion tensor imaging study investigating fractional anisotropy (FA)—a measure thought to reflect white-matter myelination and axonal integrity and coherence—found that binge drinking adolescents had lower FA than control subjects in seven frontal, three parietal, two temporal, four subcortical, and two cerebellar regions. Furthermore, in six of these regions, lower FA was associated with significantly greater lifetime hangover symptoms and higher estimated peak blood alcohol concentrations.35
In a second cross-sectional study, concomitant binge drinking and substance using adolescents had lower FA than control subjects in 10 separate frontal, parietal, temporal, and subcortical regions, and reduced FA in these regions was associated with greater lifetime alcohol use.36 Interestingly, the investigators also noted three regions (i.e., the superior longitudinal fasciculus, internal capsule, and occipital lobe) where FA was greater in concomitant binge drinking and substance using adolescents than in control subjects, and they found that greater FA in these regions was associated with greater lifetime alcohol use.
Finally, a third cross-sectional study of binge drinking adolescents and concomitant binge drinking and substance using adolescents found that binge drinking adolescents, again, had lower FA than control subjects in eight different regions, including the superior corona radiata (SCR), inferior longitudinal fasciculus, superior longitudinal fasciculus (SLF), inferior fronto-occipital fasciculus (IFOF), and cerebellar peduncle.37 Those with concomitant substance use, in contrast, only had significantly lower FA (compared with control subjects) in three regions, including the SCR and SLF, and they had significantly higher FA than binge drinking adolescents in four regions (i.e., the SCR, SLF, IFOF, and cerebellar peduncle). In this study, greater marijuana use frequency was associated with greater FA in the SCR and SLF, whereas a greater number of lifetime drinks was associated with greater FA in the SLF. Together, these findings suggest that binge drinking during adolescence is associated with reduced FA, but that concomitant marijuana use may interact with the effects of alcohol, resulting in an alteration of this effect.
These cross-sectional findings have been corroborated by numerous longitudinal studies. Luciana and colleagues reported that compared with control subjects, adolescent binge drinkers showed significantly diminished normative increases in FA in the dorsal caudate and IFOF between baseline and follow-up visit.32 Another study found that concomitant binge drinking and substance using adolescents had reduced FA in the corpus callosum, prefrontal thalamic fibers, and posterior corona radiata at follow-up, compared with control subjects, with no differences reported at baseline.38
A series of studies examined FA in a group of binge drinking and concomitant binge drinking and substance using adolescents and young adults at baseline and follow-up.39-41 First, they found that binge drinking adolescents both with and without concomitant substance use showed a significant, widespread decline in FA across the three visits, resulting in lower FA after 3 years of use compared with control subjects.39 Moreover, lower FA in the fornix and SCR at baseline in concomitant binge drinking and substance using adolescents predicted greater subsequent use at the first follow-up, above and beyond baseline substance use.40 It is important to note that in these two studies,39 adolescent binge drinkers and substance users were not drug and alcohol naïve at baseline; rather, they were drinking and using marijuana throughout the entirety of the study. Lastly, Jacobus and colleagues identified 20 regions in the brain where there was a significant group-by-time interaction, such that adolescents who used both alcohol and marijuana concomitantly showed a sharper decline in FA between baseline and 3-year follow-up than those who only binge drank.41 In combination, these findings suggest that whereas binge drinking during adolescence and young adulthood appears to be associated with reduced FA, results tend to be less clear when adolescents concomitantly use marijuana. Whereas Jacobus and colleagues found that binge drinkers with concomitant marijuana use initially had had greater FA than those who only binge drank,37 a longer history of concomitant marijuana use, extending into young adulthood, may eventually result in a steeper decline in FA across development.41
Effects on Brain Function
Verbal Encoding
Learning and memory abilities are crucial for an adolescent’s success, and development of those abilities may be altered or attenuated by alcohol use. Verbal encoding/learning, using a verbal paired-association task, has been used to investigate the impact of alcohol on learning and memory in binge drinking adolescents with and without comorbid marijuana use. A preliminary study found that binge drinking adolescents had greater activation in the SFG, superior parietal lobule, inferior parietal lobule (IPL), and the cingulate, as well as lower activation in one cluster encompassing the cuneus, precuneus, lingual gyrus, and parahippocampal gyrus (PHG) during novel word encoding.42
In a follow-up investigation, Schweinsburg and colleagues found that binge drinking and concomitant binge drinking and substance using adolescents, when compared with marijuana-only users and control subjects, showed greater encoding-related activation in the postcentral gyrus, IPL, and SFG, and less activation in the fusiform gyrus, PHG, cuneus, precuneus, IPL, IFG, precentral gyrus, and cingulate.43 They also identified regions of the brain (i.e., the IFG, miFG, SFG, and cuneus) where users of either alcohol or marijuana showed greater brain response than nonusers during novel word encoding, whereas users of both substances resembled nonusers. Because performance on the task was the same between binge drinkers and control subjects,42,43 these findings suggest that alcohol use during adolescence may cause adolescents to adopt a different neural strategy (e.g., heavier prefrontal-cortex recruitment) to achieve the same successful verbal encoding. Because of the cross-sectional design, it is unknown whether these differences were present prior to or developed as a consequence of alcohol consumption.
Working Memory
Brain response during working memory also has been shown to be altered in binge drinking adolescents and young adults. In a preliminary study, Tapert and colleagues found that brain response during a visual working memory task was negatively associated with subjective response to alcohol, such that adolescents who reported that a greater quantity of alcohol was needed to feel an effect showed greater activation in the SFG, cingulate, cerebellum, and PHG during memory retrieval.44 A subsequent study showed that binge drinking adolescents had greater activation in the medial frontal gyrus (meFG), SFG, IPL, and supramarginal gyrus, as well as less activation in the middle occipital gyrus, when compared with control subjects.45 Furthermore, in longitudinal analyses, binge drinking adolescents actually had lower activation in the IPL and meFG at baseline (i.e., prior to drinking), but when compared with control subjects, they showed a greater increase across time. These greater increases in brain activation were associated with a greater peak number of drinks in the past year, more past-month drinking days, and greater withdrawal/hangover symptoms at follow-up.45 Further, less premorbid activation in the meFG and IPL predicted a higher peak number of drinks and drinking days in the year preceding follow-up.45 This suggests that binge drinking not only affects neural response during working memory, but that baseline differences in brain activation during working memory may be useful in identifying adolescents who may go on to drink.
These findings also are supported by cross-sectional work using other working memory tasks. One study found that during verbal working memory, binge drinking young adults had greater activation in the parietal cortex (pre–supplementary motor area) than control subjects.46 Moreover, more drinks per drinking occasion were associated with greater dorsal medial PFC activation, whereas more drinking occasions per week were associated with greater cerebellar, thalamic, and insular activation. In contrast, Squeglia and colleagues reported that binge drinking adolescents had lower activation in the SFG and IFG compared with control subjects.47 However, this study differed in two ways from the previous studies. Squeglia and colleagues used a spatial working memory task and also reported significant sex differences, such that binge drinking females showed less activation than control subjects, and binge drinking males showed greater activation than control subjects in the SFG, IFG, ACC, miFG, miTG, superior temporal gyrus, and cerebellum. These findings suggest that, in general, adolescents show alcohol-related increases in activation, particularly in fronto-parietal networks during working memory; however, at least for spatial working memory, these findings may be sex specific. Further work is necessary to tease out the different elements (e.g., spatial versus verbal) of working memory and the effects of alcohol on their associated neural responses.
Risk Taking and Reward Response
Because adolescence is a time of increased risk taking, including experimentation with alcohol, it may come as no surprise that binge drinking adolescents show altered brain response during various phases of risk taking. Whereas some investigators have attempted to elucidate binge drinking’s effects on a particular aspect of risk-taking behavior,48-50 others have investigated risk taking more broadly.51 In a study looking at risk-taking behavior using the Iowa Gambling Task, binge drinking adolescents had greater risk-related activation in the amygdala and insula compared with control subjects, and they had more reported drinking problems related to less activation in the orbitofrontal cortex (OFC) and more activation in the insula.51 Two recent studies separately investigated the effects of binge drinking during adolescence during decision making and reward receipt. In the first study, binge drinking adolescents, compared with control subjects, showed reduced cerebellar response during reward receipt following initiation of binge drinking, a finding that remained significant when controlling for premorbid activation, and which was associated with more drinks per drinking day in the past 90 days.48
A longitudinal investigation found that binge drinking adolescents, compared with control subjects, had lower activation in the IFG, IPL, miTG, and superior temporal gyrus across time, suggesting a different pattern of brain activation that occurs prior to binge drinking and persists after alcohol initiation.49 There also was a significant group-by-time interaction in the dorsal caudate, such that binge drinking adolescents showed similar risky decision-making–related brain responses as controls at baseline, but they showed a reduced response following binge drinking. This reduction was associated with a greater number of drinking days and heavy drinking days in the previous 3 months.
Further, Worbe and colleagues used a novel risk-taking gambling task in binge drinking young adults to investigate brain responses during the decision-making and feedback phases of both reward and loss gambles.50 During decision making in conditions with both a low and high potential for a loss, the study found that binge drinkers had greater activation in the OFC, superior parietal cortex, and DLPFC compared with control subjects. This finding was accompanied by more risky decisions during high-loss selections. Furthermore, although giving feedback during the task reduced the amount of risky decisions in binge drinking young adults, it also was associated with greater activity in the IFG and IPL, when compared with control subjects.
In addition to studies looking at adolescent risk-taking behavior, a study by Whelan and colleagues investigated brain responses during reward anticipation and receipt outside of the context of risk, using the monetary incentive delay task.29 The study demonstrated that, compared with control subjects, adolescent binge drinkers had greater activation during reward receipt in the SFG prior to initiation of binge drinking, but they had reduced activation during reward anticipation and receipt in the ventral medial PFC and IFG after binge drinking. Taken together, these findings suggest that binge drinking during adolescence and young adulthood is associated with alcohol-related alterations in brain response during decision making and reward/consequence notification. Further, group differences in fronto-parietal brain response during risky decision making and reward receipt that occur prior to drinking may serve as a risk factor for future drinking.29,49
Inhibition
Several longitudinal studies have used a standard go/no-go procedure to investigate the effects of binge drinking on brain response during inhibition. One study found that, at baseline, adolescents who went on to engage in heavy drinking had reduced brain response during successful inhibition in the DLPFC, miFG, SFG, IFG, meFG, paracentral lobules, cingulate, putamen, miTG, IPL, and pons, compared with adolescents who remained alcohol naïve.52 In another study, less activation during successful inhibition in the ventral medial PFC predicted more alcohol dependence symptoms in heavy-drinking adolescents at 18-month follow-up.53 Meanwhile, in a study investigating the failure to inhibit responding, greater activation in the premotor cortex served as a risk factor for adolescents who later went on to engage in binge drinking.29 Together, these studies suggest that lower engagement of numerous regions, particularly within the fronto-parietal network, during successful inhibition, as well as greater engagement of premotor regions during unsuccessful inhibition, may precede the onset of binge drinking.
Furthermore, compared with alcohol-naïve control subjects, heavy-drinking adolescents were shown to have significantly lower levels of brain activation during inhibition in the miFG, IPL, putamen, and cerebellum at baseline.54 They also showed greater increases in inhibition-related brain responses, compared to controls, following initiation of heavy drinking. Greater increases in brain response during response inhibition between baseline and follow-up were associated with more lifetime drinks. The same group of researchers also found that these patterns of activation differed in adolescents who experienced alcohol-induced blackouts. Prior to initiation of heavy drinking, adolescents who did and did not experience alcohol-induced blackouts showed less activation in the IPL compared with control subjects.55 However, adolescents who went on to experience alcohol-induced blackouts showed greater activation during inhibition in the miFG, miTG, cerebellum, and parietal cortex (pre–supplementary motor area) compared with those who did not experience blackouts. These findings suggest that adolescents who later experience alcohol-induced blackouts show patterns of brain activation during inhibition, which may render them more vulnerable to the memory-impairing effects of alcohol.
Lastly, a recent study in binge drinking young adults found that those who escalated drinking over a 12-month period had greater fronto-parietal activation during inhibition compared with young adults who maintained stable drinking levels.56 Taken together, it appears that hypoactivation of the fronto-parietal network during inhibition may serve as a risk factor for alcohol use initiation; however, after alcohol use initiation, hyperactivation of the fronto-parietal network during inhibition may serve as a risk factor for escalation of drinking.
Cue Reactivity
Two recent studies have looked at brain activation elicited by an alcohol cue (i.e., cue reactivity), using an alcohol pictures task, in binge drinking adolescents and young adults. Dager and colleagues found that young adults who transitioned from moderate to heavy drinking over a 1-year follow-up had greater activation at baseline in the caudate, ACC, medial prefrontal cortex, precentral gyrus, insula, IFG, and OFC, compared with those who remained moderate drinkers or heavy drinkers throughout the study.57 Furthermore, brain activation in this network of regions predicted future drinking and alcohol-related problems, above and beyond baseline drinking characteristics. This suggests that changes in how the brain responds to alcohol cues may help predict which individuals may transition from light to heavy drinking and may be more informative than simply comparing heavy drinkers with control subjects. In another study, heavy-drinking adolescents had greater cue-elicited brain response in the dorsal striatum, cerebellum, PHG, and thalamus than control subjects prior to abstinence; however, the group differences in the cerebellum and ACC no longer remained significant after 28 days of abstinence.58 This suggests that although cue-elicited brain response may be a predictor of future drinking, if adolescents manage to maintain abstinence, they may be able to reduce that cue-elicited response. This finding has important implications for future intervention strategies.
Effects on Behavior and Cognition
Many of the structural and functional differences observed in adolescent binge drinkers also are associated with changes in cognition and behavior. Several studies have examined neurocognitive changes related to binge drinking and reported poorer performance in many domains, including attention,59,60 learning and memory,59,61-66 and visuospatial functioning.60 Neuroimaging studies have found that the poorer sustained attention observed in binge drinking adolescents is associated with thicker PFCs31 and lower FA in the inferior longitudinal fasciculus67—regions where thickness and FA differed significantly between binge drinking adolescents and control subjects. This suggests that binge drinking during adolescence may cause a delay in the maturation of both gray and white matter, resulting in poorer sustained attention.
Furthermore, binge drinking adolescents and young adults have demonstrated impaired performance on a variety of learning and memory tasks.59,61,62,64,65 These findings also have been associated with changes in brain structure in binge drinking adolescents in regions of the brain where these adolescents differ from control subjects. Binge drinking–related deficits in working memory also have been demonstrated,61,63 with one study showing that after 3 years of binge drinking, greater gray-matter volume in the DLPFC was positively associated with working-memory errors.26 Further, decreased FA in the inferior longitudinal fasciculus in binge drinking and substance using adolescents has been shown to be associated with poorer working-memory performance.67 In addition, although an initial study found that the number of drinking days in the past year predicted greater reductions in performance on a visuospatial task,60 a follow-up study showed that thicker frontal cortices corresponded with poorer visuospatial performance in binge drinking females.31 These findings suggest that delayed cortical maturation may underlie the effects of binge drinking on visuospatial performance.
Binge drinking adolescents also demonstrate impaired, or riskier, decision making,68 likely resulting from impairments in impulsivity69 and inhibition.64 One study found that young adults who showed stable, high levels of binge drinking made riskier choices on the Iowa Gambling Task compared with adolescents who engaged in stable, low levels of binge drinking.68 Other studies have reported that heavy-drinking adolescents show greater impulsivity than light drinkers69 and that binge drinking adolescents show impaired inhibition compared with control subjects.64
Neuroimaging studies have helped shed some light on the mechanisms underlying this impaired decision making and impulse control. Structurally, greater impulsivity in adolescent binge drinkers has been shown to be associated with smaller DLPFC and IPL volumes and greater dorsal cingulate and precuneus volumes,70 whereas reduced FA in the fornix of concomitant binge drinking and substance using adolescents has been shown to predict greater amounts of risky behavior a year and a half later.40 Functionally, riskier behavior on the Iowa Gambling Task in binge drinking adolescents has been accompanied by greater activation in the insula and amygdala, when compared with control subjects.51 Also, as described above, greater activation in the OFC, superior parietal cortex, and DLPFC, when compared with controls, has been associated with more risky decisions when there was a high potential for loss.50 Taken together, these findings suggest that the underdevelopment of control regions (e.g., smaller DLPFC and IPL volumes) and hyperactivation of reward-salience regions (e.g., amygdala), both of which are hallmarks of adolescent neurodevelopment, may be exacerbated in adolescents who binge drink and may underlie the observed increase in risk-taking behavior in binge drinking adolescents.
Conclusions
Although evidence is still emerging on how binge drinking during adolescence and young adulthood affects the brain, many general conclusions can be drawn from current literature (for a summary of all replicated findings in binge drinking adolescents and young adults, see Figure 1). First, binge drinking during adolescence appears to result in a decrease in both gray-matter volume and cortical gray-matter thickness,9,30 with longitudinal studies suggesting that some of these differences may be present prior to binge drinking and continue to worsen as adolescents initiate alcohol consumption.27,28,32 Although it must be noted that some studies show increased gray-matter volume or thickness in binge drinking adolescents, it is plausible that these contradictory findings either are caused by the influence of concomitant marijuana use33,34 or are the result of examining the effects of binge drinking on a nonlinear developmental pattern2-5 in a cross-sectional manner.24,26
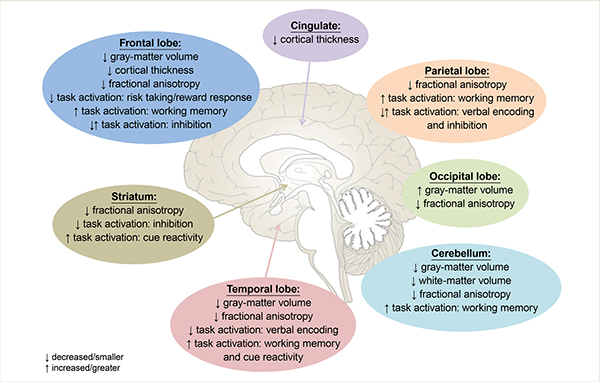
Second, multiple studies consistently have shown that the developmental increases in white-matter volume, often observed in adolescents,2,6-8 appear to be attenuated in adolescents who binge drink,27,28,32 and that this attenuation is associated with the degree of substance use.9,25 However, studies demonstrating altered white-matter microstructure in binge drinking adolescents have yielded mixed results, showing both increases and decreases in FA. Again, it appears that this may partially be explained by the presence of concomitant marijuana use in adolescence.36,38-41 More studies comparing concomitant users to those using only alcohol or marijuana likely are necessary to completely disentangle these effects.
Functionally, binge drinking during adolescence appears to affect brain responses in numerous regions, across a variety of tasks. Cross-sectional work has identified both increased and decreased brain activation in multiple task domains (e.g., verbal learning, working memory, risk taking, cue reactivity, and inhibition) and demonstrates the necessity of longitudinal studies to determine which effects are a result of alcohol consumption and which reflect an underlying risk phenotype for those who will go on to binge drink. Longitudinal work, specifically in working memory45 and response inhibition,52,54 suggests that binge drinking adolescents demonstrate similar or lower levels of brain activation in task-relevant regions at baseline, followed by an exacerbated increase in activation, above and beyond that seen in control subjects, after initiation of binge drinking. A failure to recruit task-relevant regions at baseline in future binge drinkers could lead to poorer task performance, while hyperactivation following alcohol use suggests that binge drinking adolescents require more recruitment of task-relevant networks to achieve desired cognitive outcomes.
Meanwhile, similar or lower levels of brain activation during risk-taking behavior (i.e., risky decision making and reward response) also have been observed in binge drinking adolescents.48,49 However, unlike during working memory and response inhibition, binge drinking adolescents have lower levels of brain response over time during risky decision making and reward response. This may suggest not only a pattern of activation during risky decision making that may serve as a risk factor for future drinking,49 but also a diminished brain response to risky stimuli and rewards following binge drinking.48,49 This decreased brain response may be what causes binge drinking adolescents to show greater risky behavior and may enhance reward seeking.
Understanding these altered neurobiological features in binge drinking adolescents is extremely relevant, because changes in both brain structure and function have been related to changes in cognition in binge drinking adolescents.26,31,40,50,51,60,67,70 Moreover, not only do differences in task activation serve as risk factors for future drinking,45,49,52,54 but neurobiological features, such as fronto-parietal hyperactivation during inhibition and atypical white-matter microstructure, may serve as risk factors for escalated drinking and risk-taking behavior in adolescents who are already drinking.40,56 Adolescent onset of alcohol use has been associated with an increased risk for developing an AUD later in life;22 thus, understanding neurobiological markers that are associated with both initiation and escalation of alcohol use is important for advancing future prevention and intervention strategies in an effort to reduce the rates of AUD.
Disclosures
The authors declare that they have no competing financial interests.

