Volume 43, Issue 1 ⦁ Article Number: 01 ⦁ https://doi.org/10.35946/arcr.v43.1.01
Abstract
PURPOSE: Although abstinence is recommended in pregnancy, many pregnancies are exposed to alcohol. Observational studies of the effects of low to moderate prenatal alcohol exposure (PAE) and neurodevelopmental outcomes have yielded inconsistent results, with some studies finding an increased risk of adverse neurobehavioral and cognitive outcomes, and other studies finding no changes or reduced risk of the same outcomes. The purpose of this narrative review is to summarize these inconsistencies and apply a methodological framework to discuss how different parameters contribute to the findings. The authors also provide recommendations on how to advance future research in this area.
SEARCH METHODS: The PubMed, Web of Science, and Embase databases were searched, along with reference lists of selected systematic reviews and meta-analyses. Search terms used were (infant or child or children or adolescent or offspring) AND (low or light or mild or moderate or low-to-moderate) AND (drinking or alcohol or drinks) AND (pregnancy or prenatal or fetal) AND (neurodevelopment or behavioral or psychological or cognitive or developmental) NOT (mice or rat or fish or animal) NOT (meta-analysis or review). Peer-reviewed original research studies were included if they analyzed associations between an exposure defined and characterized as low/light or moderate PAE with offspring neurodevelopmental outcomes. Animal studies, studies that did not provide clear cutoff points to classify PAE categories, studies lacking an abstinence control group, and studies that did not present a multivariable-adjusted measure of association were excluded.
SEARCH RESULTS: The searches identified 2,422 papers, with 36 papers meeting eligibility criteria. These studies were carried out across nine countries and included samples ranging from approximately 500 to 40,000 participants. Cognitive, academic, socioemotional, and behavioral outcomes were assessed from infancy through age 19.
DISCUSSION AND CONCLUSION: When the findings from the selected articles were summarized by geographic region, exposure definition, or neurodevelopmental outcome, no consistent observations or patterns emerged between low to moderate PAE and offspring outcomes. Although some studies found positive (i.e., beneficial) associations between low to moderate PAE and outcomes (primarily outcomes related to cognition) and others found negative (i.e., detrimental) associations (primarily for behavioral outcomes), most findings were null (i.e., showed no effect of PAE). The heterogeneity in study results is likely due to methodological issues, including residual confounding, effect measure modification, and exposure misclassification that make synthesis of studies difficult. Alternative study designs, including longitudinal trajectory analysis, sibling design, negative controls, and instrumental variable analyses, may reduce biases and are discussed. To date, the consequences of light to moderate levels of PAE on neurodevelopment remain unresolved; studies that advance methodological rigor will be important contributions to the field.
Introduction
Prenatal alcohol exposure (PAE) is a necessary cause of fetal alcohol spectrum disorders (FASD), a group of alcohol-related conditions characterized by neurodevelopmental problems. Although PAE is associated with many adverse physical, neurodevelopmental, and social outcomes, the most commonly studied are neurodevelopmental—primarily behavioral and cognitive—outcomes. Associations between heavy PAE (which is inconsistently defined) or binge PAE (defined as consuming four or more drinks in about 2 hours in women, or the amount of alcohol necessary to achieve a blood alcohol concentration of 0.08% or higher1) and adverse neurodevelopmental outcomes have been well documented in the literature.2-4 However, findings regarding associations between lower levels of PAE and neurodevelopmental outcomes are inconsistent, with summations to date yielding, at best, inconclusive results.5 Moreover, there is no consensus in the literature on the definition of “low to moderate” PAE—or, correspondingly, the level of harm that low to moderate PAE may cause5—leaving pregnant individuals and their clinicians ill-equipped to assess risk of exposure.
Several systematic reviews6-10 and at least four meta-analyses2,11-13 have assessed associations between low to moderate PAE and child neurodevelopmental outcomes. Pooling results from studies published through 2012, Flak and colleagues reported a small positive association between mild to moderate PAE (defined as up to six drinks per week) and child cognition (beta estimate 0.04; 95% CI [0.00, 0.08]; seven studies).2 They also identified a modest association between moderate PAE (defined as up to six drinks per week, including some individuals who consumed at least three drinks per week) and adverse behavioral outcomes, such as problems with behavior regulation and increased demand for attention at ages 9 months to 5 years (beta estimate -0.15; 95% CI [-0.28, -0.03]; three studies).2 A more recent meta-analysis, pooling studies published through 2020, also found that low to moderate PAE (author characterized, or one to fewer than seven drinks per week) was associated with adverse behavioral outcomes (i.e., attention problems) at ages 6 to 17 (OR 1.21; 95% CI [0.88, 1.65]; six studies). However, the magnitude of associations estimated varied dramatically across studies.11 Dissimilar to the prior studies, a meta-analysis specifically examining the effect of low to moderate PAE (≤ 20 g/week to ≤ 50 g/week) on risk of attention-deficit/hyperactivity disorder (ADHD) reported no effect (OR 0.96; 95% CI [0.86, 1.02]; six studies).12 Studies included in this review used a few different measures to assess ADHD symptoms between ages 3 and 14.12
The impetus remains to better understand the relationship between low to moderate PAE and offspring neurodevelopmental outcome. Although most authoritative bodies recommend complete abstinence from alcohol during pregnancy, PAE continues to be common, particularly in the early weeks of gestation prior to pregnancy recognition. In surveys conducted by the Behavioral Risk Factor Surveillance System between 2018 and 2020 in the United States, about 14% of pregnant women reported past 30-day alcohol use.14 It is possible that the inconclusiveness in previous research findings is not driven by a paucity of research, but by inconsistencies in methodology used across studies. The purpose of this narrative review is thus threefold. First, it briefly summarizes select literature of low to moderate PAE and neurodevelopmental outcomes, noting consistencies and inconsistencies in findings. Second, it reviews methodological issues that limit valid ascertainment of the effects of low to moderate PAE on offspring neurodevelopmental outcomes. Third, it discusses alternative study designs that may address key methodological issues for consideration in future research.
Methods
Search Strategy
The PubMed, Embase, and Web of Science databases were searched on June 13, 2022. The search terms used to identify articles were (infant or child or children or adolescent or offspring) AND (low or light or mild or moderate or low-to-moderate) AND (drinking or alcohol or drinks) AND (pregnancy or prenatal or fetal) AND (neurodevelopment or behavioral or psychological or cognitive or developmental) NOT (mice or rat or fish or animal) NOT (meta-analysis or review). Results of the search strategy were checked against reference lists of existing systematic reviews and meta-analyses to verify that the search was comprehensive.2,6,11,12
Eligibility Criteria
The inclusion criteria for this review were: (1) peer-reviewed original research study; (2) human participants; (3) PAE characterized as low, light, mild, moderate, or low to moderate; and (4) any neurobehavioral or developmental outcome in offspring. Exclusion criteria were: (1) reference group other than “no PAE” or abstinence; (2) no parameters provided for the quantity or frequency of alcohol exposure that was used to classify individuals as having low, light, mild, moderate, or low to moderate PAE; (3) inability to separate PAE from co-occurrence with exposure to other substances; (4) no adjustment for confounding variables; (5) no measure of association presented for low/light or mild/moderate PAE categories (e.g., exposure was analyzed as a continuous variable); (6) quasi-experimental study design; and (7) alcohol exposure not specific to the pregnancy period.
Data Abstraction and Synthesis of Results
One of two reviewers examined titles and abstracts of each article to identify articles meeting criteria for full text review. During full text review, one of two reviewers abstracted the following information from each study: (1) author and year; (2) data source and sample size; (3) study setting and offspring birth years; (4) definition of low/light PAE; (5) definition of mild/moderate PAE; (6) definition of one drink or one unit of alcohol; (7) timing of PAE measurement; (8) outcome, outcome measurement tool, and age of offspring at outcome measurement; (9) confounding variables considered; and (10) results. These data are summarized in Appendix 1.
Results
The search of PubMed (n = 1,644), Embase (n = 455), and Web of Science (n = 1,040) databases yielded 2,422 unique records, 65 of which met criteria for full text review. Of these, 29 were excluded (see Figure 1 for details of exclusions), and 36 were included in the final set. With a few exceptions, most studies reported no associations between low or moderate PAE and offspring neurodevelopment. Appendix 1 synthesizes the results by study location, exposure definition, timing of exposure measurement, and neurodevelopmental outcome.
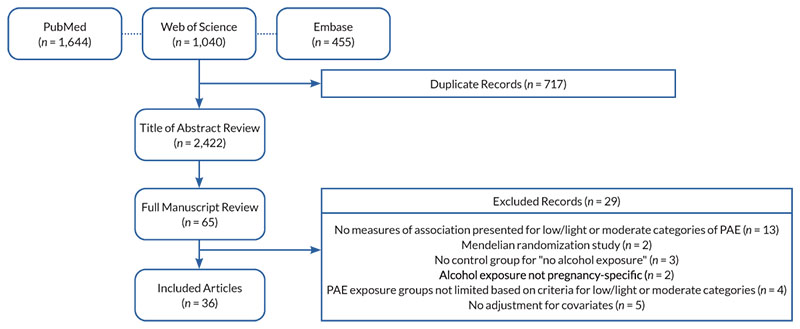
Figure 1. Sample selection for inclusion in this review. Note: PAE, prenatal alcohol exposure.
Study Location
Included studies reported data from Australia (eight studies);15-22 New Zealand, Australia, Ireland, and the United Kingdom (one study);23 Denmark (nine studies);24-32 Denmark and Finland (one study);33 Japan (one study);34 South Africa (one study);35 the United Kingdom (nine studies);36-43 and the United States (six studies).44-49 Of note, many of the studies published from the same country used data from the same source. For example, half of the Australian studies used the Western Australian Health Survey data;15,16,19,21 seven of the nine Danish studies used data from the Lifestyle During Pregnancy Study (based on a sample from the Danish National Birth Cohort);26-32 and six of the nine U.K. studies used data from the Millennium Cohort Study.36-39,41,50 Individual studies with the same data source often used the same definition of PAE but varied by study outcome or offspring age at neurodevelopmental assessment.
Some patterns were observed in study findings by country. For example, all studies from Denmark and Denmark/Finland reported mostly no effect of low to moderate PAE (i.e., null findings). Two studies—one using data from the Danish National Birth Cohort25 and one using data from the Aarhus Birth Cohort24—published findings suggesting potential protective associations between low PAE and ADHD in both sexes24 and between low to moderate PAE and internalizing problems among boys.25 Results from each of the Australian studies were also mostly null, although two studies reported some evidence for worse behavioral problems with low PAE,15,17 and three studies reported some evidence for better behavioral,16 cognitive,18 and academic outcomes with low PAE.21 Studies from the United Kingdom focused on potential sex differences in the associations between low to moderate PAE and neurodevelopmental outcomes. Two studies using data from the Avon Longitudinal Study of Parents and Children reported worse behavioral rating scores among girls (but not boys) exposed to light PAE compared to children without PAE.40 Two analyses using data from the Millennium Cohort Study reported that light PAE was associated with better cognitive scores36,37 and lower behavioral scores37 among boys relative to boys without PAE. Of the six studies from the United States, findings from three studies suggested worse behavioral outcomes with light to moderate PAE compared with no PAE,45,46,48 one study noted a possible protective association between light PAE and autism spectrum disorder (ASD),44 and the remaining two studies showed null results47,49 (see Appendix 1).
Prenatal Alcohol Exposure
There was tremendous heterogeneity in how low/light or moderate PAE were defined. Most studies included in this review defined these categories based on the average quantity of drinks consumed per week. Of these, low/light PAE was most frequently defined as averaging one to four units of alcohol per week, with specific definitions ranging from less than one unit per week to less than seven units per week. The studies using the higher threshold of fewer than seven units per week were mostly conducted in Australia, matching the definition of low-risk drinking for women.15-17,20,21,52 Many studies also considered units per occasion in their drinking definition. For example, most studies using data from the Millennium Cohort study defined low PAE as “not more than one to two units per week or per occasion;”36-38,50 and most studies using the Western Australian Health study defined low PAE as “not more than seven drinks per week and up to two drinks per occasion.”15,19,20,21,52 The study from South Africa considered only the number of drinks per occasion (mild to moderate: not more than three drinks in one sitting, never binge drinking),35 and the study from Japan considered only frequency of drinking (low PAE: drinking rarely to one to four times per month).34 For moderate PAE, most definitions were between three and 10 drinks per week. Some considered drinks per occasion in addition to drinks per week (range from more than two to five drinks per occasion).
The definition of a drink or unit of alcohol also varied by country. A standard drink was typically defined as 10 grams of pure alcohol in Australia; 12 grams in Denmark; and 8 grams or half a pint of beer, a glass of wine, or a single measure of spirits or liquor in the United Kingdom (see Appendix 1). No clear pattern of study findings emerged by definition of low/light PAE, moderate PAE, or unit of alcohol.
Timing of PAE Assessment
In all studies analyzed, PAE was assessed using self-report by the women as there are no biomarkers that could provide details on the dose and timing of low to moderate PAE. Twenty-two of the included studies collected self-report information on PAE during pregnancy,16-18,20,22-33,35,40,42,43,47,49 and 14 studies relied on reports obtained after pregnancy,15,19,21,34,36,37,39,41,44-46,48,50 including 10 studies with reporting occurring within 1 year of delivery.15,19,21,36-39,41,45,50 Only three studies assessed PAE at the same time as or after the infant outcome was assessed (i.e., retrospectively). Of these, two reported that light to moderate PAE was associated with worse behavioral problems at age 23 months48 and at ages 9 to 10 years;46 and the third—a case control study examining associations with ASD—reported mostly null findings, with the exception of a protective association between one to two drinks in the first month of pregnancy and lower odds of ASD.44
Neurodevelopmental Outcomes
The studies included in this review assessed neurocognitive, academic, and behavioral outcomes when offspring were between the ages of 9 months and 19 years. None of the included studies reported worse neurocognitive outcomes with low to moderate PAE. For example, studies examining associations between PAE and Bayley Scales of Infant Development mental development and psychomotor indices in infants ages 12 to 24 months reported mostly null findings,17,20,42,52 with one study suggesting that low PAE in the second and third trimesters was associated with better cognitive outcomes.18 Three studies examining associations between PAE and IQ at about age 5 also reported null findings.23,26,29 Four studies using data from the Millennium Cohort Study that assessed cognitive development with the British Abilities Scale reported protective associations between light PAE and cognitive outcomes in boys at age 336 and age 5,37 but null associations when the boys were evaluated at ages 7 and 11.38,39
For measures of socioemotional and behavioral health, most studies used data ascertained with either the Strength and Difficulties Questionnaire (10 studies25,30,33,36-41,43) or the Child Behavior Checklist (CBCL; seven studies15,16,22,23,34,35,46). For these measures, offspring age at the time of assessment varied greatly and results were mixed with no clear pattern identified. Others studies examined PAE in relation to specific diagnoses, including ADHD (two studies)24,41 and ASD (two studies),44,50 with all studies reporting null or protective results. Three studies used infant behavior rating checklists to measure behavioral outcomes in infants ages 9 to 24 months. All three studies reported that light to moderate PAE was associated with worse behavioral outcomes in infancy, including increased infant difficultness,48 poorer social engagement,45 and more sensation seeking.17
Regarding academic outcomes, three studies reported null associations between PAE and academic achievement.39,40,49 One study reported that low PAE was associated with lower odds of meeting Australian numeracy academic benchmarks, but had no effect on meeting reading, spelling, or writing benchmarks.21
Discussion and Comment
Despite a large and growing evidence base, making inferences about the effects of light to moderate PAE on neurodevelopmental outcomes in offspring remains difficult. Heterogeneity in effect estimates has persisted over the past 20 years, with both harmful and protective effects observed. Due to differences in study approach (summarized in Appendix 1) and methodological limitations, the diverse study results are difficult to synthesize. The following section further details these issues.
Methodological Issues With the Study of Light to Moderate PAE
Definition of exposure
Currently, there are no standard criteria or consensus for defining low/light or moderate levels of PAE. This heterogeneity in exposure definitions has undoubtedly contributed to inconsistencies of effect estimates and has limited the ability to make comparisons between studies. Even in abstracting data for this review, the authors were limited to papers that self-classified exposure into low/light or moderate PAE. Undoubtedly, many additional studies have investigated similar exposure levels but did not label them as such and therefore could not be included in this review because they were not identified in the literature searches.
Nondifferential exposure misclassification: Static measurement of PAE
Studies of PAE often use time-insensitive, or static, categorizations of exposure (e.g., categorizing the entire pregnancy as “high” consumption based only on PAE at conception) that fail to incorporate the dynamic changes in exposure that occur across pregnancy. These changes typically occur around the time of pregnancy recognition (which can be highly variable across individuals) when many women reduce their consumption or abstain from alcohol. For some pregnancies, changes also occur later in pregnancy as the perceived “risk period” for fetal development passes and women feel more comfortable resuming some level of alcohol use. The timing of these transition points may be informative with respect to offspring development but frequently is not examined. Further, when time-varying exposures are collapsed into static variables, a resulting exposure misclassification may lead to attenuation of effect estimates. This is particularly of concern when studying the consequences of low and moderate PAE, where modest effect estimates can disappear entirely due to exposure misclassification.
Differential exposure misclassification: Recall bias
There are no validated biomarkers that reflect prenatal exposure several years after birth; consequently, PAE is frequently measured by maternal recall. The gold standard approach in the collection of PAE information is through timeline follow-back53 during pregnancy before the outcome is known, after which recall bias may occur. Due to stigma, individuals who perceive neurobehavioral problems in their offspring may be more likely to underreport their prenatal alcohol consumption relative to individuals who perceive no problems, which in cases of extreme underreporting could result in estimated protective effects of PAE. Although it is much more feasible to collect information retrospectively once children are at suitable ages for neurodevelopmental evaluation, the impact of this approach on internal validity must be critically evaluated.
However, when assessing studies that rely on recalled PAE, research has noted that recalled alcohol exposure is strongly predictive of pregnancy, dysmorphic, and neurodevelopmental outcomes.54 Further, validation studies comparing prospective and retrospective reports found that retrospective reports of maternal drinking reflect higher levels of consumption than prospective reports obtained during the prenatal period.55-58 Thus, although collection of consumption data during pregnancy remains preferable, retrospective information should not be discounted simply due to potential recall bias, and actually may be more accurate in some groups, particularly those who perceive stigma when reporting during pregnancy. Notably, however, the ability to recall PAE after pregnancy may differ by level of prenatal alcohol use. Women who did not consume any alcohol as well as those who habitually had high levels of consumption both may have more accurate recall than women who infrequently consumed alcohol, particularly with respect to the precise timing and amount of alcohol. This differential exposure misclassification could move estimates of PAE effects in either direction (toward or away from the null).59
Confounding variables
To validly estimate a causal effect of PAE on neurodevelopment, the exposure groups must be interchangeable. This means that variables that could confound the association between light to moderate PAE and offspring outcomes are equally balanced between alcohol-exposed and non–alcohol-exposed offspring. In observational studies, many socioeconomic and psychosocial factors have been associated with alcohol consumption patterns. A study of more than 6,000 women in Australia examined maternal factors associated with patterns of alcohol consumption before, during, and after pregnancy. The analysis found that compared to women with light prenatal alcohol consumption (0.4 drinks per day pre-pregnancy, early pregnancy cessation), women with high levels of alcohol consumption (2.5 drinks per day pre-pregnancy, 0.6 drinks per day during pregnancy) were more likely to have a lower income, be single or divorced, be pregnant for the first time, not attend church, report depression or anxiety, have high maternal adversity, and have adverse health-related lifestyle behaviors (e.g., smoking, little exercise, poor sleep).60 Within the same study, women who abstained from alcohol pre-pregnancy through postpartum were also more likely to have lower income, have more children, and have adverse health-related lifestyle behaviors compared to women with light prenatal consumption.60 Similarly, in a study of 4,000 pregnant women in the United Kingdom, women of higher socioeconomic status were more likely to drink wine, which was more likely to be consumed in low to moderate amounts, and less likely to binge drink than those with lower socioeconomic status. Further, being older, being better educated, having a higher social class, being employed, and having a better educated, employed partner were associated with consumption of wine, whereas smoking, lower education, and worse mental health were more strongly associated with consumption of beer.61 Other factors that favor positive neurobehavioral outcomes in the offspring were disproportionally shared by women who consumed low to moderate amounts of alcohol. These factors include better diets, earlier use of prenatal vitamins, lower prevalence of mental illnesses such as depression or anxiety, and lower likelihood to use other substances (e.g., marijuana) during pregnancy.62 These studies highlight the imbalance in protective factors that often accompany low to moderate PAE that may bias findings, resulting in null or even protective effects when compared to abstinence.
Although these and other factors associated with low to moderate prenatal alcohol consumption that reduce the likelihood of adverse neurodevelopmental outcomes are routinely included in multivariable adjustment, they may still result in unmeasured confounding. Additionally, sample sizes typically limit the ability to include the multitude of variables necessary to establish true exchangeability. As a result (and common in all observational studies), the degree to which residual confounding persists—as evidenced by positive associations often reported between low to moderate PAE and offspring neurodevelopmental outcomes—must be considered because there is no conceivable benefit on these outcomes from PAE itself.63,64
Effect modification
In addition to confounding, which affects the internal validity (bias) of an estimate, the effect modification of the estimate by factors associated with the outcome (which can be termed modifiers or moderators) must be considered. Although not a source of bias, this occurs when the magnitude of the effect varies across levels of a third variable, which may contribute to heterogeneity in effect estimates across studies. Here, the authors hypothesize higher socioeconomic status associated with low to moderate PAE to be the third variable. For example, women consuming low to moderate levels of alcohol in pregnancy, which as previously noted is associated with higher socioeconomic status, may have more resources at their disposal postnatally compared to either women who abstain or women who consume large quantities of alcohol, providing a more enriched environment for the offspring. These factors include the likelihood to breastfeed, high-quality childcare, reduced environmental exposures, higher levels of social support, access to health care, reduced caregiver stress, and educational resources available to the child, which benefit neurodevelopmental outcomes. When effect estimates for PAE are not stratified by these potential modifiers, outcomes most vulnerable to PAE may be masked by the preponderance of protective factors associated with low to moderate PAE.
In summary, the null or protective effects attributed to low to moderate PAE on neurodevelopmental outcomes are likely, at least in part, attributable to unmeasured confounding associated with the exposure as well as to effect modification by postnatal factors that favor children with low to moderate PAE. Many statistical tools exist to address confounding (e.g., propensity score adjustment, inverse probability of treatment weights) and effect modification (e.g., stratification, re-weighting to a standardized sample). However, these options are imperfect because of the strong psychosocial patterning of PAE and insufficient resources to validly measure all potential confounding variables or examine associations across population subgroups. Further, as previously noted, there is a high degree of nuance in the operationalization of PAE, highly likely leading to misclassification of exposure and attenuation of results. To overcome these challenges, researchers have begun applying novel study designs and exposure models to this research. The following section highlights a few such approaches.
Alternate Study Designs to Address Methodological Issues
The study designs and methodologies discussed in this section—which are by no means exhaustive—specifically target some of the aforementioned threats to validity. Thus, exposure misclassification can be addressed by determining longitudinal trajectories of PAE, and confounding can be limited through use of instrumental variables, sibling designs, and negative control studies. These methods can be used alone or in combination to enhance estimation of causal effects.
Longitudinal trajectories of PAE
Efforts to better capture and operationalize the three parameters of alcohol use (timing, dose, and duration) have been ongoing for many years. Initially, researchers manually clustered individuals based upon these characteristics. In one study, investigators created a composite measure of PAE that incorporated dose, pattern, and timing of consumption into a descriptive, categorical variable (e.g., low, moderate, binge drinking less than once per week; binge drinking once or twice per week; high PAE).15 When they compared outcomes using these classifications to traditional analytic methods (i.e., average quantity per trimester, average daily exposure across pregnancy, average weekly exposure across gestation), the researchers detected increased odds of anxiety or depression for children with moderate PAE in the composite models that were not evident in the traditional analyses.15 Lately, unsupervised machine learning techniques have been incorporated into analyses to identify patterns of use across gestation.
Although several methodologies exist, the underlying premise of longitudinal exposure modeling is to create groups with similar longitudinal exposure patterns to minimize heterogeneity within the assigned trajectory group and maximize heterogeneity between trajectories. In a study employing PAE trajectories in Ukraine, sustained alcohol exposure, even at relatively low levels (about one drink per day), was associated with modest reductions in neurodevelopmental performance at 6 and 12 months of age compared with trajectories of higher PAE with alcohol reduction or cessation earlier in pregnancy.65 In the Safe Passage Study (South Africa and the United States), the sustaining trajectory (modeled as maximum drinks per drinking day) was associated with sudden infant death syndrome, whereas the trajectories with similar early pregnancy consumption but earlier cessation were not.66 By modeling trajectories, researchers can disaggregate patterns of consumption, even among individuals with low to moderate levels of consumption, to better identify and understand the nuanced risk of adverse offspring outcomes that are often lost when exposure variables are operationalized as static measures.
Instrumental variables
Instrumental variable (IV) analysis is another study design that could improve research on low to moderate PAE and address issues of exchangeability. An IV affects the outcome only through its effect on the exposure and is unrelated to potential confounders of the exposure–outcome association.67 In experimental studies, the treatment assignment is the IV. However, when experimental studies are not feasible, researchers may use a ”quasi-experimental” approach to an IV through ”Mendelian randomization,” involving the use of genetic variants that influence the exposure but are unrelated to factors that confound the exposure-outcome relationship.68 Three recent studies using data from the Avon Longitudinal Study of Parents and Children (ALSPAC) utilized this design.61,62,68 In one study, investigators used analysis of a maternal genetic variant in the alcohol dehydrogenase gene ADH1B as an instrument for assessing PAE.61 Individuals who carry the rare variant rs1229984 in ADH1B have greatly increased enzymatic activity in the oxidation of ethanol to acetaldehyde. This increased activity results in a faster reduction of blood alcohol levels and sharper rise of acetaldehyde in blood and organs, leading to symptoms such as increased heart rate and nausea. Individuals with this variant consume less alcohol, and correspondingly, the fetuses of mothers with the variant have lower PAE. These observations were born out in the ALSPAC data, making this a suitable IV candidate for low to moderate PAE.61,62,68 When the researchers used a traditional analysis of the effects of any alcohol exposure, estimates were largely null, indicating no effect. In contrast, when they stratified the analysis by type of exposure (i.e., preferred type of beverage), they noted positive effects between wine consumption and offspring academic achievement, and negative effects between beer consumption and offspring academic achievement. Given that there should be no difference between the effects of beer and wine when both were converted into standard, equivalent doses, the researchers suggested that the different outcomes were due to the strong social gradient associated with choice of beverage. ADH1B, however, was unrelated to potential confounders, but was predictive of alcohol use in pregnancy and, by extension, alcohol exposure to the developing fetus. When authors conducted the IV analysis with ADH1B, they found negative effects between PAE and academic achievement at all ages analyzed (i.e., ages 7 to 16).61 Similar results were obtained when repeating this analysis with cognitive and educational performance at age 8.68
The third study that utilized an IV analysis constructed the IV from the child’s genotype (variants of the alcohol dehydrogenase enzyme). It was hypothesized that offspring alleles, which result in “fast” metabolism of ethanol, would protect against abnormal brain development in infants.62 Among offspring born to women with moderate alcohol consumption (one to six units per week during pregnancy), negative associations existed between the presence of genetic variants associated with slow alcohol metabolism and IQ at age 8. These associations were not seen in children with no PAE.62 Assuming these findings can be replicated, they are a promising avenue to control for confounding factors while estimating the effects of low to moderate PAE on child health. Researchers should continue to explore additional IVs, specifically searching for stronger IVs than the alcohol metabolism variants, which can be exploited in this framework.
Sibling controls
A third promising approach that also addresses issues of exchangeability is a sibling design. In their simplest form, sibling designs compare outcomes among exposure-discordant siblings, which accounts for many shared environmental and familial confounders that are exceedingly difficult to fully adjust for through multivariable analysis. Sibling studies often result in attenuated effect estimates between prenatal exposures and offspring neurodevelopmental outcomes compared to traditional study designs,69 highlighting the challenges of residual confounding.
At least three studies have utilized sibling designs to control for shared genetic and environmental confounders when studying PAE.34,70,71 In the first sibling study conducted on approximately 4,000 mother-sibling triads, researchers found that employing the sibling design attenuated the initial multivariable-adjusted results for PAE and attention/impulsivity problems in offspring. However, an association still existed between heavy PAE (≥ 5 days/week) and offspring conduct problems at ages 4 to 11.70 In a second study, conducted with 15,000 mother-sibling triads in Norway, researchers detected no effects of low levels of PAE on offspring behavioral problems, and attenuated yet modest effects of “hazardous” PAE on behavioral problems at age 3 when accounting for siblings.71 A third study, which included 1,600 sibling pairs in Japan, found that low PAE (drinking one to four times per month or rarely) was associated with greater anxiety problems and internalizing problems. In that study, effect estimates were magnified in the siblings analysis compared to initial multivariable-adjusted results.34 Although researchers must consider exposure discordance and factors that changed between births (e.g., birth order, maternal age, socioeconomic factors) that may bias results, this model is a promising strategy to mitigate the persistent problem of residual confounding.
Negative controls
Finally, another option that does not require identifying an IV or observing siblings is to employ a negative control design. Such designs compare the effects of PAE on offspring outcome with the effects of similar exposure with no biological relevance to the offspring (e.g., maternal exposure prior to conception or postnatally or similar levels of exposure of other individuals [partner exposure]).67 Negative control designs alert the analyst to uncontrolled confounding because if any of the effect estimates among the negative controls are positive, the effect measure of interest is likely confounded. Putting this design to practice, researchers using paternal exposure during pregnancy as a negative control in the Avon Longitudinal Study of Parents and Children found no evidence that maternal alcohol and tobacco consumption during pregnancy were more strongly associated with childhood IQ than paternal alcohol and tobacco consumption.72 In a second negative control study using ALSPAC data, researchers found that offspring of mothers who consumed any alcohol at 18 weeks of gestation had a 17% increased risk of having a diagnosis of depression at age 18.73 There was no clear evidence of association between partners’ alcohol consumption at 18 weeks of gestation and increased risk of offspring depression. The investigators concluded that the negative control comparison of paternal drinking provided some evidence that the association between PAE and depression at age 18 may be causal and warranted further investigation and replication.73 Although one must critically evaluate the potential for causality by the selected negative control (e.g., epigenetic effects in the case of paternal exposure), if it is deemed that there is no plausible mechanism by which the negative control could affect the outcome, the types of analyses should be conducted and reported.
Conclusions
Limitations of This Review
When reviewing the findings presented here, several limitations should be considered. First, restricting findings to published research introduces the potential for publication bias. However, given that many studies reviewed here yielded null findings, it is unlikely that publication bias greatly affects the results. Second, the high degree of heterogeneity in methods and the exposure definition prevented a meta-analysis.
Third, and most importantly, although three databases were searched, this review is a narrative review only and should not be interpreted as a systematic review. The search process used search terms “low, light, mild, or moderate,” and the authors then looked for predefined criteria for the categorization. Accordingly, there could be additional studies that included similar exposure ranges but did not use those terms; other studies may have included a population with a mean PAE that fell within a range described as low or moderate, but the study did not actually set minimum or maximum parameters for defining the exposure. Either scenario would have resulted in exclusion of those studies from this review. For example, Parry et al. examined several categories of alcohol exposure (i.e., 0 g/week, > 0–29 g/week, 30–59 g/week, 60–89 g/week, 90–119 g/week, and ≥ 120 g/week)74 that overlap with definitions of low/light or moderate PAE in many of the studies included here. However, the study was excluded because the authors did not label these categories as low, moderate, or high exposure. Similarly, Beauchamp et al. enrolled a birth cohort with a PAE group that had a median of 0.84 ounces absolute alcohol per day during the periconceptual period and 0.3 ounces absolute alcohol per day during pregnancy.75 Although this sample may have overlapped with other samples in this review of moderate PAE, it likely also included infants who would have been categorized as low PAE and some who would have been categorized as high PAE, given that no minimum or maximum criteria were set for inclusion. Therefore, the study was excluded from this review. Attempting to include all such studies would require (1) searching for any study that had any measure of PAE, and (2) having clearly defined and accepted standards for categorizing low and moderate PAE. Such manual abstraction and classification in the absence of consensus definitions were beyond the feasibility of this review.
Fourth, the studies reviewed here assessed a variety of neurodevelopmental outcomes that may differ in sensitivity to alcohol based on the measure or the timing of administration. It was beyond the scope of this review to comment on each measure’s sensitivity and psychometric properties, although both also may contribute to the heterogeneity in findings. Finally, this review was limited to neurodevelopmental outcomes. There are other health and behavioral outcomes potentially affected by PAE that manifest across the life course and also warrant systematic investigation.
Public Health and Clinical Implications
Although pregnant women may want to know whether there is a safe drinking threshold in pregnancy, this question is difficult to answer in human research. As seen in this review, findings are heterogeneous across studies, and many methodological limitations impair ability to validly estimate the potential consequences of low to moderate PAE. Moreover, the public should not confuse inconsistent evidence and insignificant findings to indicate absence of an effect. Accordingly, the only way to be certain to avoid adverse outcomes associated with alcohol exposure is to follow current guidelines to abstain from alcohol during pregnancy. Given that many women do not plan pregnancy, those who have had alcohol exposure prior to learning they are pregnant should avoid continued use.
Summary and Future Directions
Although this review generally found null associations between low to moderate PAE and adverse neurodevelopment, the issue is far from resolved. There is no consensus in the literature on the level of harm that low to moderate prenatal alcohol exposure may cause, and the differential vulnerability resulting from influences such as concurrent exposures to other substances, genetics, and other factors may prevent a clear conclusion. Although the evidence base continues to expand, substantial methodological limitations impair synthesis of study findings. Use of alternative study designs may help advance research of the effects of low to moderate PAE on adverse outcomes, and the authors look forward to the expansion of these methodologies in the field. In addition, expanding reviews to capture other outcomes, including physical and behavioral outcomes, as they emerge across the life course is of great interest.
Acknowledgments
Gretchen Bandoli is funded by an NIH award (K01 AA027811). The authors would like to acknowledge the contributions of Madison Chapman in the quality control process for this paper.
Correspondence
Address correspondence concerning this article to Gretchen Bandoli, 9500 Gilman Drive, MC 0828, La Jolla, CA 92093. Email: gbandoli@health.ucsd.edu
Disclosures
The authors declare no competing financial or nonfinancial interests.
Publisher's note
Opinions expressed in contributed articles do not necessarily reflect the views of the National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health. The U.S. government does not endorse or favor any specific commercial product or commodity. Any trade or proprietary names appearing in Alcohol Research: Current Reviews are used only because they are considered essential in the context of the studies reported herein.

